Historical Perspectives of Industrial PTC Process Development Over the Past Decades
As today is the last day of the decade of the 2010’s, it is interesting to reflect on changes we have seen over the past few decades in PTC process development. We would like to share here some general conclusions about trends we found in the segmentation of the PTC process development market, though we will not disclose proprietary details about our customer base. We will also share historical perspectives about PTC technology development in industry and academia.
The following discussion is based on qualitative observations as well as an analysis of internal data at PTC Organics regarding the segmentation of phase-transfer catalysis process technology development customers. We looked at the industries and the geographical location of our customers’ process development headquarters.
 First, we have a qualitative idea of shifts in the market segments and location of PTC process development efforts that we observed by our market presence as the leader in PTC process development in the past quarter century. Personally, I have been a full-time industrial phase-transfer catalysis entrepreneur with PTC Organics Inc. (and its predecessor PTC Communications Inc.) since 1995 and I have seen structural changes in the organic chemical industry like many of you have seen. With PTC Organics Inc. (and previously PTC Communications Inc.), I have attended and/or exhibited at tradeshows such as Informex, CPhI and Chemspec in each of the past 25 years and I have witnessed the shifts in industrial organic chemical activity on a qualitative basis. In addition, we analyzed the sales of PTC Organics’ over the past 20 years since companies that actively develop PTC technology turn to PTC Organics Inc. to address their most challenging phase-transfer catalysis projects to achieve lower cost higher performance green chemistry. When companies invest real money in PTC technology development, that shows commitment to the technology.
First, we have a qualitative idea of shifts in the market segments and location of PTC process development efforts that we observed by our market presence as the leader in PTC process development in the past quarter century. Personally, I have been a full-time industrial phase-transfer catalysis entrepreneur with PTC Organics Inc. (and its predecessor PTC Communications Inc.) since 1995 and I have seen structural changes in the organic chemical industry like many of you have seen. With PTC Organics Inc. (and previously PTC Communications Inc.), I have attended and/or exhibited at tradeshows such as Informex, CPhI and Chemspec in each of the past 25 years and I have witnessed the shifts in industrial organic chemical activity on a qualitative basis. In addition, we analyzed the sales of PTC Organics’ over the past 20 years since companies that actively develop PTC technology turn to PTC Organics Inc. to address their most challenging phase-transfer catalysis projects to achieve lower cost higher performance green chemistry. When companies invest real money in PTC technology development, that shows commitment to the technology.
Early Days of Phase-Transfer Catalysis
When I first entered phase-transfer catalysis in 1976, five years after Charles Starks’ classic paper in which he coined the term phase-transfer catalysis, the majority of the breakthrough work in PTC was published by researchers in the US and Europe, mostly by academics, though Starks was an industrial chemist himself and there were already industrial PTC processes in Europe.
The manufacture of tetrabutylammonium bromide (TBAB) started when DuPont had a commercial PTC process that needed TBAB and they turned to surfactant-quat producer, Hexcel in Zeeland, Michigan, to produce TBAB. Sachem (in Texas) were making high purity quats for a variety of applications (including tetramethyl ammonium hydroxide for the new semiconductor industry). In the early 1980’s, Sachem and Zeeland Chemicals were the main ammonium quat producers in the US for small quats with up to about 20 carbon atoms and Henkel and Witco were the producers of Aliquat ® 336 and Adogen ® 464 with an average of 27 carbon atoms. Cytec (Canada) made aliphatic quaternary phosphonium salts and still do. Parish Chemical was the first commercial producer of 18-crown-6 and dibenzo-18-croen-6 in the US. In Europe, Dynamit Nobel was the main producer of TBAB and related quats in the early days and Rhone Poulenc produced TDA-1 which was an excellent complexant phase-transfer catalyst. In 1988, Dishman in India became a major producer of quaternary ammonium phase-transfer catalysts and grew their business significantly over the next decade by aggressive marketing.
By the time I completed my Ph.D. work in phase-transfer catalyzed hydroxide ion reactions in 1983, PTC technology development in academia had a strong presence in the US, Germany, Israel, Sweden, Italy, Poland and India. When I started presenting industrial PTC lectures around the world in 1995, PTC was thriving in the global chemical industry with many commercial applications growing in all industrialized countries, notably adding Switzerland, Japan, the UK, the Netherlands, Austria and Spain that enjoyed strong growth using PTC to manufacture products in a wide variety of industries. Though not well known publicly, there was much industrial PTC growth in Russia, as Dr. Felix Sirovski shared in the journal Phase-Transfer Catalysis Communications in 1997. Dr. Sirovski wrote that the robustness of PTC technology for a very wide range of commercial applications was an excellent match for the chemical plants in Russia that did not always enjoy investment in plant maintenance that was common in the West.
In 1987, Catalytica commissioned a study about the past, present and future of industrial phase-transfer catalysis and gathered the experts, Charles Starks of Vista Chemical (formerly of DuPont and Conoco), the inventor of PTC and the extraction mechanism; Charles Liotta of Georgia Tech and top notch award winning consultant at companies such as DuPont and Milliken; Dan Brunelle of General Electric, inventor of many PTC patents for the manufacture of engineering thermoplastics and inventor of several thermally stable phase-transfer catalysts, Howard Alper of University of Ottawa who was the leader in transition-metal PTC technology and myself, Marc Halpern, then a process chemist at Dow Chemical that was already manufacturing hundreds of millions of dollars of polymers, agrochemicals and pharmaceuticals using phase-transfer catalysis. I authored the study sold by Catalytica that included a study of 125 PTC patents up to that point.
In 1994, Charles Starks, Charles Liotta and I published the authoritative 650-page book “Phase-Transfer Catalysis: Fundamentals, Applications and Industrial Perspectives.” A year later, I founded PTC Communications Inc. and in 1999, I founded PTC Organics Inc. and co-founded PTC Value Recovery Inc.
2000-2009
By the year 2000, I presented the lecture “Reducing Cost of Manufacture of Organic Chemicals Using Phase-Transfer Catalysis” at more than 100 industrial process R&D departments in North America, Europe and Asia. By the year 2000, it was clear that the penetration of phase-transfer catalysis reached every segment of the chemical industry, not just the classical pharmaceuticals and agrochemicals. A study on PTC published in 2019 by non-PTC experts still perceived (incorrectly) that pharma and agrochem were the major users of phase-transfer catalysts. PTC is a highly technical niche and it is hard for outsiders to understand the market by casual study.
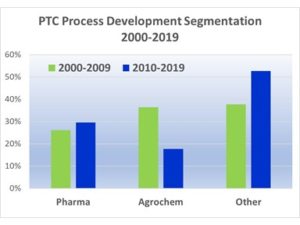
In the first decade of the 2000’s, large industrial PTC processes were growing or newly commercialized for a wide variety of monomers, polymers, fragrances, solvent, petrochemical applications and environmental applications in addition to the classical pharma, agrochem and specialty/fine organic chemicals. During the decade of 2000-2009, the investment of chemical companies in PTC process development for non-pharma/non-agrochem products was 38% of the total.
In the decade of the 2000’s, Asia-Pacific countries (most notably Japan, India and China) had great growth in PTC applications in all industrial segments. One of the greatest breakthroughs in all of phase-transfer catalysis is the family of “Maruoka chiral phase-transfer catalysts” invented by Keiji Maruoka in Japan starting just before the year 2000 and grew in patented applications in the 2000’s.
2010-2019
In the decade ending today (Dec 31, 2019), PTC process development continued to grow in all industrial segments and in all geographies. In fact, PTC Organics had a great year in 2019 in terms of sales for process development and contract research in a surprisingly wide range of applications including petrochemicals, commodity chemicals and veterinary products.
In this decade, non-pharma/non-agrochem investment in PTC process development grew to 53% from 38% in the previous decade.
In this decade, investment in PTC process development was led by European companies, followed by Asia-Pacific. Investment in PTC process development in North America remains strong, but not as strong as in Europe and Asia-Pacific. This confirms the qualitative shifts we have seen at chemical industry tradeshows over the past 25 years.
There has been a great proliferation of manufacturers of phase-transfer catalysts in India and China in the past two decades. I visited a plant in China in 2007 that produced and had in inventory nearly 50 different quaternary ammonium and phosphonium phase-transfer catalysts (many specialty phosphonium salts are used in the manufacture of epoxy resins). These days, when we obtain quotations for quaternary ammonium salts, there are many sources for these compounds, though we often have to sort through who are the actual manufacturers and who are the distributors (visits to actual manufacturing facilities).
In 2019, I conducted my 56th 2-day course “Industrial Phase-Transfer Catalysis.” That will likely be the last public PTC course I will conduct though demand for the in-house PTC course remains solid as companies recognize that phase-transfer catalysis delivers low-cost high -performance green chemistry for a very wide variety of strong base reactions, nucleophilic substitutions, oxidations, reductions, acid-catalyzed reactions and other applications in almost every industry that produces organic chemicals and polymers.
I have enjoyed a fascinating 43.5 years in phase-transfer catalysis (so far!) providing PTC services on-site at nearly 300 process R&D departments in 39 countries. Amazingly, we are now involved in what may become the largest PTC process.
The growth of industrial phase-transfer catalysis continues. Make sure that your company is not left behind. Now contact Marc Halpern of PTC Organics to explore how your company can achieve low-cost high-performance green chemistry to improve your company’s profitability and process R&D efficiency.