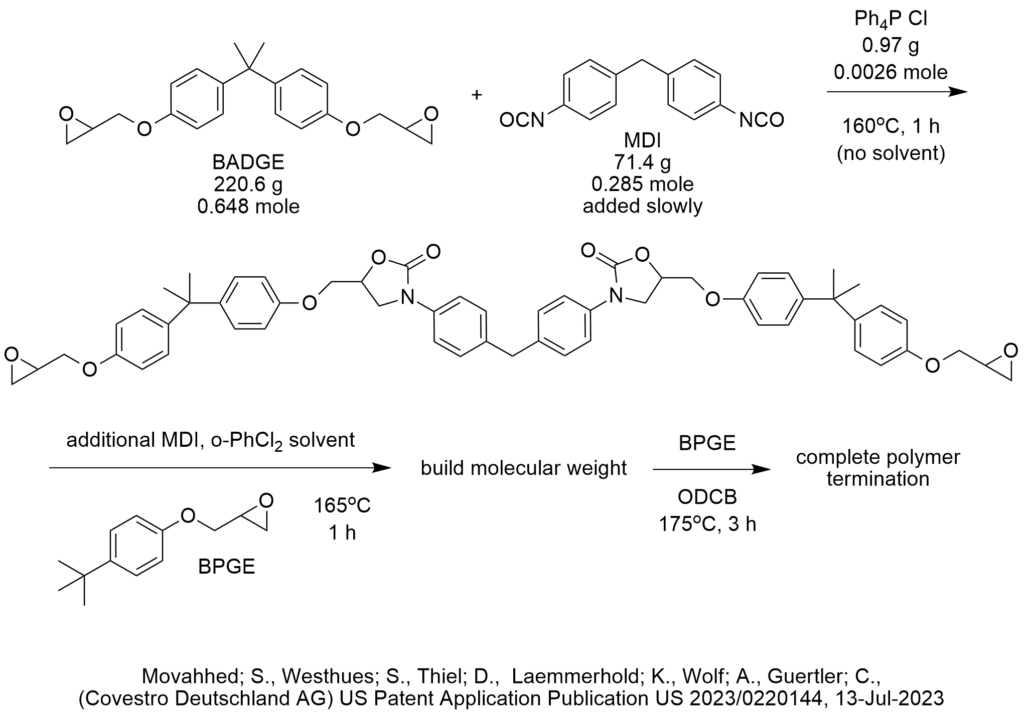
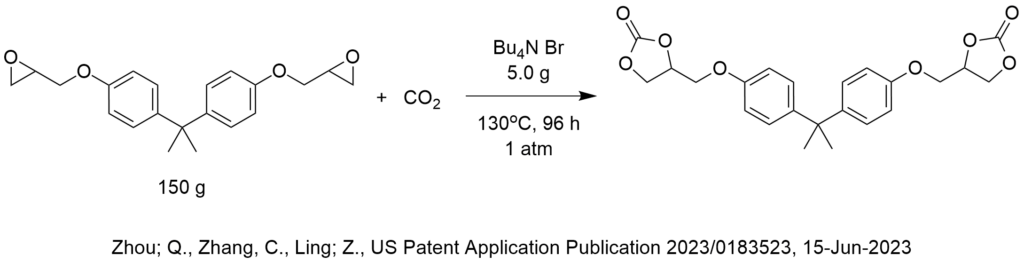
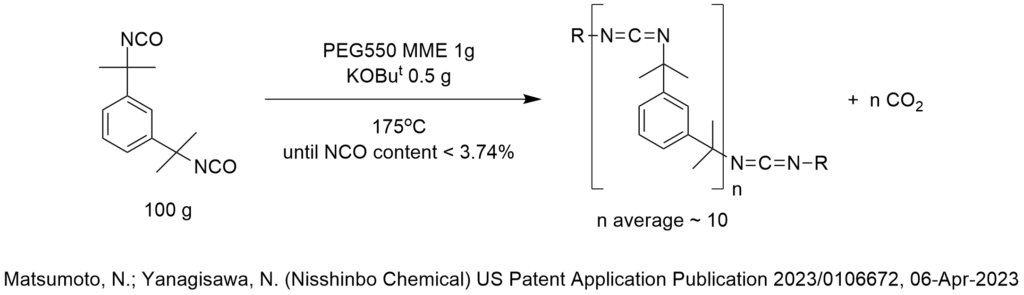
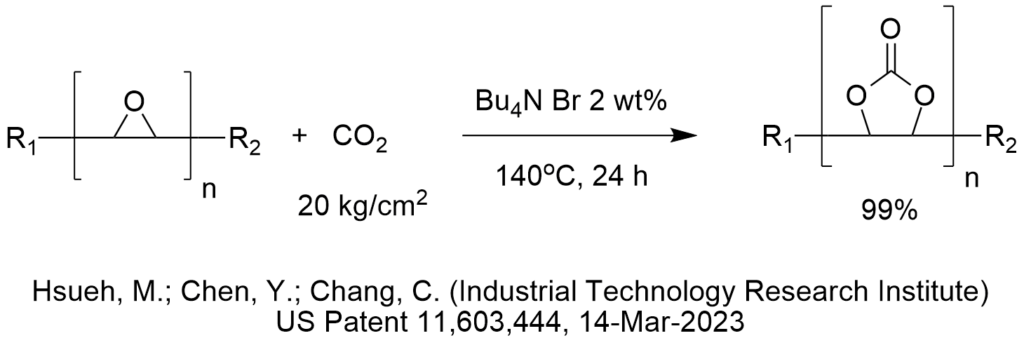
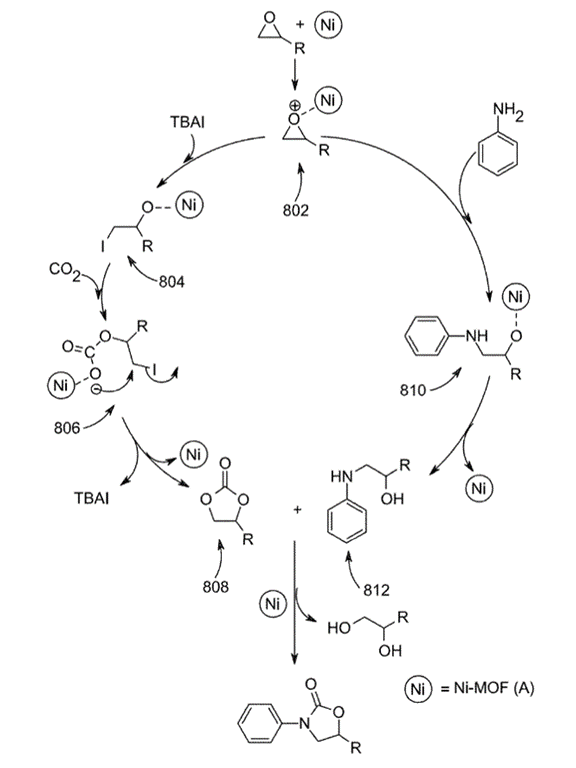
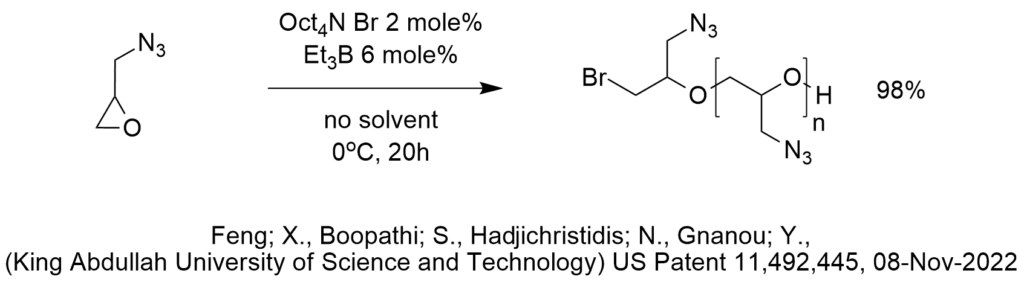
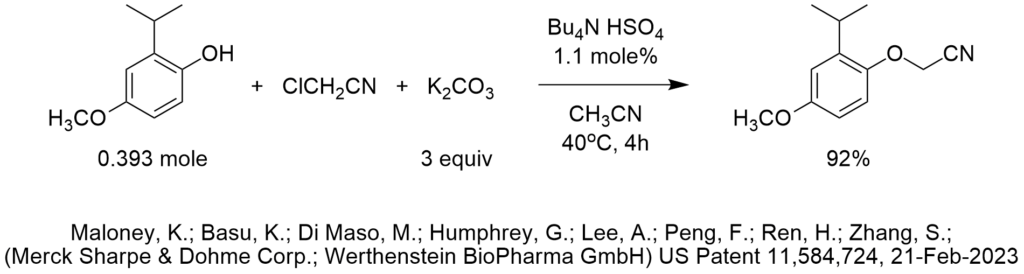Is ChatGPT a PTC Expert?
No, not yet. Not even close, yet.
How do we know that? We conducted a simple test shown below.
First, let’s provide some background on what phase-transfer catalysis information is FREELY AND PUBLICLY AVAILABLE on the internet for any artificial intelligence bot to analyze.
PTC Organics alone has published 732 blog articles critiquing PTC reactions that are freely available to AI on the internet since 2002 of which 372 are easily found right here on this website, www.PhaseTransferCatalysis.com.
ChatGPT is an artificial intelligence program that has access to everything on the internet including more than 10,000 patents published by the US Patent and Trademark Office that use tetra butyl ammonium bromide as a phase-transfer catalyst for probably every conceivable PTC reaction one can imagine. There are many more patents that cite all the other phase-transfer catalysts.
So, there is a lot of information out there that describes phase-transfer catalysis reaction conditions and the corresponding results that a computer should be able to analyze then make recommendations to choose phase-transfer catalyst, solvent, base, temperature, agitation and other reaction and process conditions.
Not only that, I (Marc Halpern) personally and explicitly pointed out in these PTC Tip of the Month blog articles (fully available to ChatGPT), hundreds of cases in which PTC reaction conditions were chosen in a NON-OPTIMAL manner. In fact, part of my contribution and value to the global PTC community was to invest a lot of my personal time, money and expertise since 1994 to publish the Industrial Phase-Transfer Catalysis Newsletter (in print!) with an initial distribution list of 10,600. I even uploaded to the internet almost all of my print PTC newsletters, that again, provides large amounts of information about choosing PTC reaction conditions.
In light of the super ability of AI to absorb all of this publicly information about phase-transfer catalysis, I decided to ask ChatGPT two simple questions about phase-transfer catalysis.
As you will see below, ChatGPT did a nice job identifying whether PTC can be used to perform a nucleophilic substitution, but it didn’t do very well when asked to choose reaction conditions.
In fact, if a chemist used the recommendations of ChatGPT as a starting point for a PTC process development project, instead of using their own expertise and skills in general process development for organic reactions, the development project would take MORE time, not less time.
Following are the questions asked and the responses from ChatGPT. Let’s see if you can identify the problems with the recommendations from ChatGPT.
========================
Question #1 posed to ChatGPT
Can one use phase transfer catalysis to perform nucleophilic substitution of 2-bromoalkanes with cyanide?
ChatGPT Answer
Yes, phase transfer catalysis can be used to perform nucleophilic substitution of 2-bromoalkanes with cyanide. In this reaction, a phase transfer catalyst is used to transfer the nucleophile, which is typically a cyanide ion, from an aqueous phase to an organic phase, where it can react with the electrophilic 2-bromoalkane. This can help to increase the reaction rate and improve the overall efficiency of the reaction.
Question #2 posed to ChatGPT
What reaction conditions should be used to perform nucleophilic substitution of 2-bromoalkanes with cyanide using phase transfer catalysis?
ChatGPT Answer
To perform nucleophilic substitution of 2-bromoalkanes with cyanide using phase transfer catalysis, the following reaction conditions should be considered:
Temperature: The reaction should be carried out at a moderate temperature, typically between 0-50°C.
Solvent: The reaction is typically performed in a biphasic system, consisting of an aqueous phase and an organic phase. The organic phase can be any polar solvent, such as acetonitrile or tetrahydrofuran, which can dissolve both the substrate and the quaternary ammonium salt phase transfer catalyst.
Base: A strong base is typically added to the aqueous phase to generate the nucleophile (cyanide ion) from the corresponding acid. Potassium hydroxide or sodium hydroxide are commonly used bases.
Phase transfer catalyst: A quaternary ammonium salt, such as tetrabutylammonium bromide, is typically used as the phase transfer catalyst. The amount of phase transfer catalyst used is usually in the range of 0.1-1.0 mol%.
Stirring: The reaction mixture should be stirred to ensure good mixing and to facilitate mass transfer between the two phases.
Time: The reaction time can vary depending on the substrate and the reaction conditions, typically between 1-4 hours.
Workup: After the reaction is complete, the two phases should be separated and the organic phase can be dried over anhydrous sodium sulfate, and then evaporated to dryness. The crude product can be purified by column chromatography or distillation.
==========
There are several issues with these recommendations. Can you identify the issues?
Hint #1: See this month’s PTC Reaction of the Month.
Hint #2: There are issues related to the fact that the reactant is a secondary alkyl bromide.
The point is that we still need thinking human chemists to choose practical process conditions.
The best way to learn how to choose PTC process conditions like an expert is to conduct the 2-day course “Industrial Phase-Transfer Catalysis” in-house at your company site. Now inquire with Marc Halpern of PTC Organics about bringing the course “Industrial Phase-Transfer Catalysis” to train the chemists at your company!
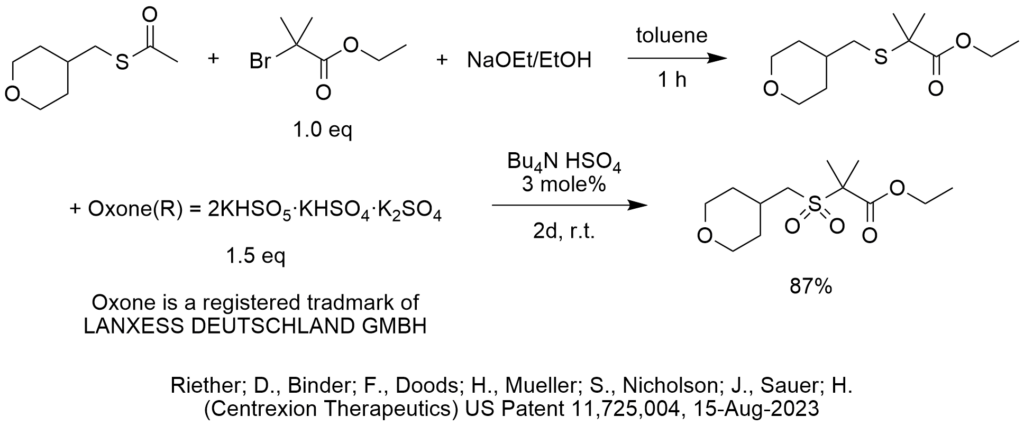 The second reaction shown in the diagram is an oxidation of a sulfide to a sulfone using Oxone® (registered trademark of Lanxess Deutschland). Oxone® is a mixture of salts including the active potassium peroxymonosulfate, which is more stable than potassium peroxymonosulfate by itself.
The second reaction shown in the diagram is an oxidation of a sulfide to a sulfone using Oxone® (registered trademark of Lanxess Deutschland). Oxone® is a mixture of salts including the active potassium peroxymonosulfate, which is more stable than potassium peroxymonosulfate by itself.