I “fell in love” with phase-transfer catalysis at first sight 41 years ago as a second year undergraduate student when I saw that you can replace expensive alkoxides with NaOH. It still bothers me after all these years when I see people wasting money. If you are a process chemist, you probably feel the same way and the reaction choices shown in the diagram probably bother you right now.
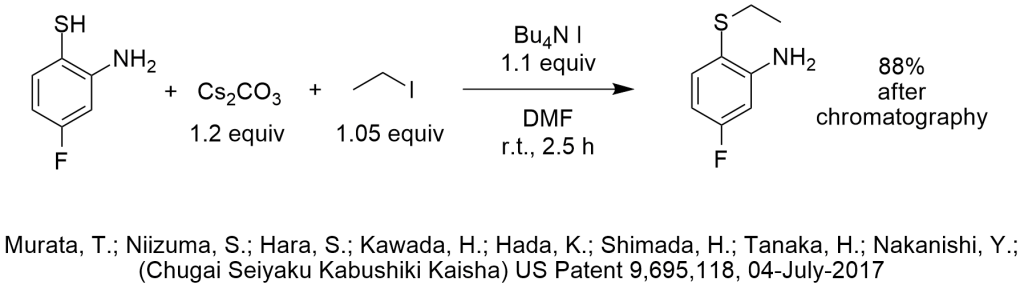
Do you know anyone who thinks they need cesium carbonate to deprotonate a thiophenol? We wouldn’t even use potassium carbonate for this reaction, we would just use 1.00 equiv of aqueous NaOH to form the sodium salt and we may even use enough water to fully solubilize the thiophenoxide. This is done in MANY PTC-thiophenoxide applications. The inventors knew enough about PTC to use a tetrabutylammonium salt for this reaction, so they must have seen at least one PTC S-alkylation in the literature.
Do you know anyone who thinks they need a full equivalent (or more!) of a phase-transfer catalyst to perform a simple S-alkylation of the highly nucleophilic thiophenoxide, an anion that has been studied extensively as a model reaction for nucleophilic substitutions to study PTC conditions and mechanisms starting in the 1970’s? I would be embarrassed to use as much as 10 mole% of a phase-transfer catalyst for this application, even during the screening stage of such a reaction.
Do you know anyone who thinks they need to use the most expensive tetrabutylammonium salt (TBAI) to do this reaction?
Do you know anyone who thinks they need to use ethyl iodide for this reaction, when ethyl bromide works well? The first PTC S-ethylation of thiophenoxide was reported in 1975 as one of the early classic publications (by Herriott and Picker). The yield using ethyl bromide was 93% in 15 minutes at room temperature and used 6% NaOH as base with 6 mole% phase-transfer catalyst…in the days before anyone knew how to optimize choice of phase-transfer catalyst.
I can understand that maybe the inventors didn’t want the ethyl bromide to evaporate (b.p. 38 C), but these reactions are fast at room temperature and don’t need a pressure vessel. Maybe there is competition from N-ethylation of the amino group, though I doubt it if we deprotonate the thiol, especially in the presence of water that would hydrogen bond to the amino group.
Do you know anyone who knows about PTC who feels they must use DMF as a solvent for a room temperature reaction when using a phase-transfer catalyst? Wouldn’t you just use toluene? Even Herriott and Picker used benzene back in the days when that was an acceptable choice of solvent.
I know that not everyone is a process chemist and that many chemists need to just make the compound with a high probability of success the first time to meet their project deadline. I understand that.
But such reports still bother me. Maybe I should see a psychotherapist. Maybe you and I will go together and get a group rate.
Now contact Marc Halpern of PTC Organics if you strongly prefer to develop low-cost high performance green chemistry processes using phase-transfer catalysis instead of seeing a psychotherapist.

