PTC excels in enabling high-performance reactions of water-sensitive compounds in the presence of water. The underlying concept that is leveraged to achieve this valuable benefit is to protect the water-sensitive compound from water by dissolving it in a water-immiscible (“water-rejecting”) organic phase using the phase boundary as a barrier, then using the phase-transfer catalyst to transfer a reactant from the aqueous phase (or solid phase) into the reaction phase in which the water-sensitive compound is dissolved.
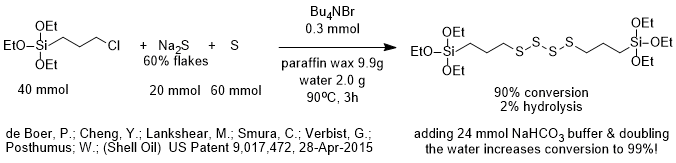
The Si-OEt group is susceptible to hydrolysis. Reactions of chloropropyltriethoxysilane (CPTES) are often performed under anhydrous conditions to avoid hydrolysis of the ethoxysilane group. The patent described here illustrates how careful choice of reaction conditions can increase the conversion of a water sensitive silane to desired product while minimizing hydrolysis.
In a patent issued earlier this week, the inventors reacted sodium sulfide and sulfur with CPTES to produce a tetrasulfide bridged bispropyltriethoxy silane (TESPT). Sodium sulfide obviously doesn’t readily dissolve in organic solvents that are easy to recover, so one would like to add some water to the system to dissolve some sodium sulfide and use a phase-transfer catalyst to transfer and react the sulfide anion from a small aqueous phase into an organic phase in which CPTES is located and protected from the water.
The inventors examined several process parameters to maximize the desired reaction and minimize hydrolysis and the results are shown in Table 3 in the patent. In all cases, they chose to use paraffin wax as the solvent, presumably to serve as a highly non-polar solvent that would be very effective in rejecting water from the reaction phase and protect the CPTES from hydrolysis.
The baseline conditions for the PTC reaction are shown in the diagram. They include using 0.8 mole% TBAB as phase-transfer catalyst, 1 part paraffin wax per part of CPTES by weight, 0.2 parts water per part of CPTES by weight and a temperature of 90 deg C. Under these conditions 90% of the CPTES is converted into bispropyltriethoxysilane tetrasulfide (TESPT) and 2% of the CPTES is hydrolyzed.
A conversion of 90% to TESPT was also observed at the higher temperature of 100 deg C while doubling the TBAB loading to 1.6 mole%. However, when increasing the temperature to 110 deg C, conversion to TESPT decreased to 80%.
A conversion of 90% to TESPT was also observed by reducing the paraffin wax loading by 60% to 0.4 parts paraffin wax to 1 part CPTES at 1.6 mole% TBAB loading. But when further reducing the paraffin wax “protecting solvent” by 73%, conversion to desired product decreased to 80%. In other words, there is a limit for how much you can reduce the solvent protection of the water-sensitive compounds.
These results suggest that process conditions must be carefully chosen to minimize hydrolysis and that means avoiding too high of a temperature and providing enough protecting solvent to minimize what may be assumed to be interfacial hydrolysis of the CPTES by water (and the Si-OEt in the product).
It is most interesting to read that when the inventors added sodium bicarbonate as buffer, they were able to achieve 99% conversion to TESPT even while doubling the amount of water to 0.4 parts water per part of CPTES by weight. This shows that it is extremely important to carefully choose reaction conditions when performing PTC reactions with water-sensitive compounds!
The inventors also tried to replace TBAB with tetraoctyl ammonium bromide and the conversion to desired product decreased into the 50’s.
At PTC Organics, we have nearly four decades of working with water-sensitive reactants under PTC conditions. Your company may be able to greatly benefit from this highly specialized expertise to increase yield of desired product and minimize excess expensive water-sensitive reactant by bringing in PTC Organics to work with you through PTC Process Consulting or PTC Contract Research. Now contact Marc Halpern of PTC Organics to explore a path forward for improving your process performance.