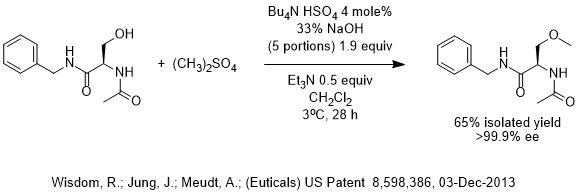
A major challenge in this patent was to use a base strong enough do the etherification but not so basic to cause racemization at the slightly acidic C-H near electron withdrawing groups. Prior technology used expensive silver oxide with methyl iodide as the methylating agent and racemization was avoided. Alternatively, sterically hindered protection of a nearby nitrogen by a trityl group could be used to partially shield the acidic C-H group from attack by base. Instead, the inventors chose to use PTC to reduce the energy of activation and reduce the reaction temperature to minimize racemization.
This patent, issued earlier this month, calls for a reaction temperature of under 20 C and adding the base in portions as it is consumed to avoid overly basic conditions. One should note that 33% NaOH is used and we can speculate that they may have tried 50% NaOH, but that it may have been “too active” and possibly caused some racemization (this is just speculation). Triethylamine was also used and forms a quat in-situ that activates the already active dimethyl sulfate. In other words, the inventors stacked the deck in their favor with every trick they could to reduce the temperature and minimize deprotonation/racemization at the slightly acidic C-H.
If you or your company can benefit from achieving higher process performance in a shorter development time for this PTC reaction or any other reaction, by having access to the best PTC expertise available, NOW CONTACT Marc Halpern to inquire about using phase-transfer catalysis to achieve low-cost high-performance green chemistry. Remember, PTC excels in thousands of reactions in more than 30 reaction categories including strong base reactions, nucleophilic substitutions, oxidations and reductions!
If you’re not sure if PTC can help your reaction, now fill out the PTC Project Evaluation Form and E-mail a scanned copy to Marc Halpern or send it by fax to Dr. Halpern at +1 856-222-1124. If your company does not have a secrecy agreement with PTC Organics Inc. already in place, please use “R-groups” instead of the exact chemical structures.