As we teach in our 2-day course “Industrial Phase-Transfer Catalysis,” the combination of PTC with NaOH is very effective for both deprotonating fluorene and activating the fluorenyl carbanion for C-alkylation.
The very first PTC reaction that I (Marc Halpern) ever performed was in 1976 and achieved quantitative yield of 9,9-dideuteriofluorene by the deprotonation and deuteration of fluorene using 50% NaOD in D2O, benzene as the organic solvent and triethyl benzyl ammonium chloride as the phase-transfer catalyst for 1 hour at room temperature. The conversion of fluorene to deuteriofluorene without a phase-transfer catalyst was 0%.
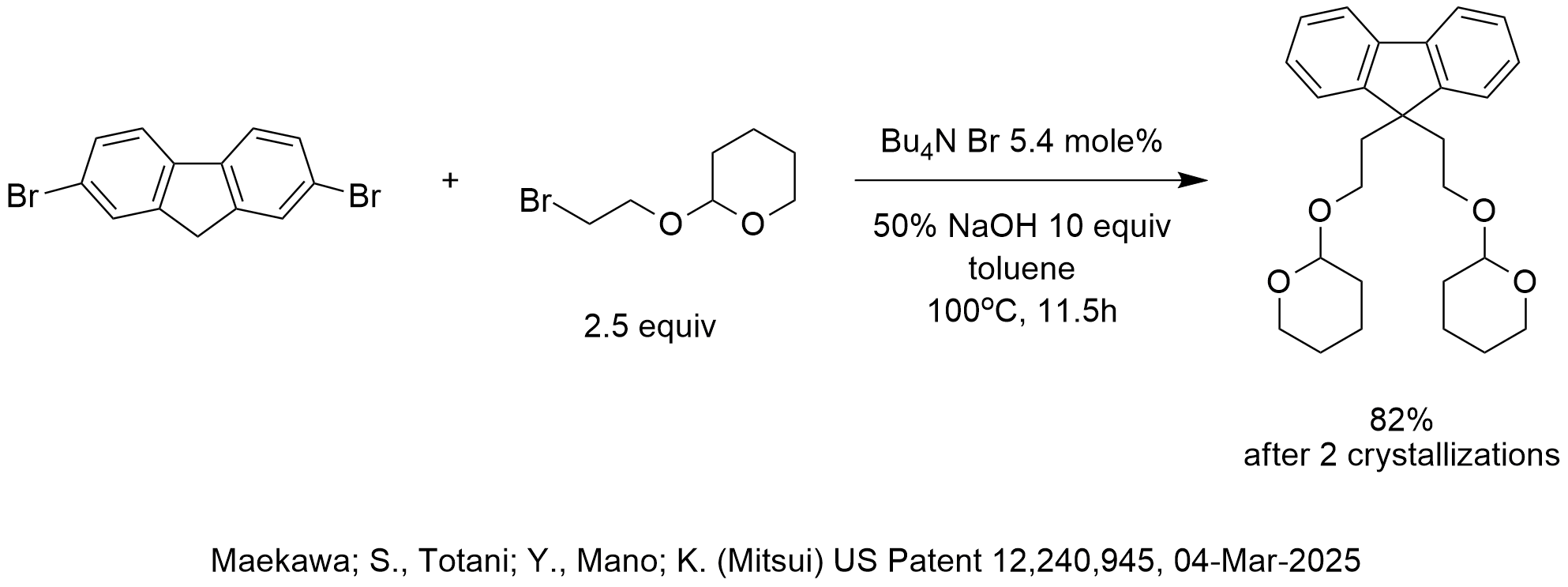
The pKa of the acidic hydrogen at the 9-position of 2,7-dibromo-9H-fluorene is approximately 21-22, based on the pKa of fluorene being 23.
In the reaction shown in the diagram, 2,7-dibromo-9H-fluorene was deprotonated and reacted with 2.5 equivalents of 2-(2′-bromoethoxy)tetrahydropyran for 11.5 hours at 100oC in the presence of 5.4 mole% tetrabutylammonium bromide and toluene as the solvent to afford the di-C-alkylated product in 82% yield after workup that included a first crystallization and a second recrystallization.
As we teach in our 2-day PTC course, according to the Halpern pKa Guidelines for the Evaluation and Optimization of New PTC Applications, since the pKa of the substrate is in the range of 16-23, then this PTC reaction is likely transfer rate limited (“T-Reaction”). According to these guidelines, an “accessible” quaternary ammonium salt is likely the best choice for phase-transfer catalyst which have a q-value in the range of 1.5-2.0. Accordingly, a better phase-transfer catalyst for this reaction might be methyl tributyl ammonium chloride (MTBAC) that has a q-value of 1.75, assuming that this quat salt would be stable enough to survive the strong base conditions and heat history. TBAB has a q-value of 1.0 and is usually less effective as a phase-transfer catalyst for T-Reactions.
Based on my experience, it might be possible to perform this C-alkylation at a lower temperature and shorter reaction time when using MTBAC instead of TBAB. If a lower heat history could be achieved for this reaction, MTBAC could be stable enough to complete the reaction.
If your company has not yet conducted the in-house course “Industrial Phase-Transfer Catalysis,” or if it has more than 10 years since you brought this course in house, you should contact Marc Halpern of PTC Organics to inquire about conducting this PTC course at your company in 2025.