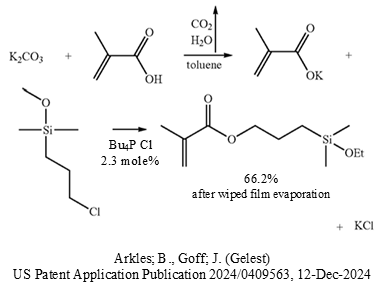
A solid-liquid PTC esterification of methacrylic acid was performed in two consecutive steps in one pot.
First, potassium methacrylate was formed from potassium carbonate and methacrylic acid in the presence of BHT stabilizer and dried with azeotropic removal of water and generation of carbon dioxide from the carbonate and water.
In the second step, without isolation of the intermediate methacrylate salt, the phase-transfer catalyst was added which was only 2.3 mole% tetrabutylphosphonium chloride. The phosphonium quat may have been chosen due to the relatively high temperature of refluxing toluene that would likely have decomposed the less expensive quaternary ammonium phase-transfer catalysts.
The water-sensitive 3-chloropropyldimethylethoxysilane was added and the esterification proceeded at reflux for 3 hours. The KCl salt byproduct was removed by filtration and the product was purified by wiped film evaporation. The yield was 66.2%.
The procedure reported was as follows: A 2 L 4-neck flask was equipped with a mechanical stirrer, heating mantle, addition funnel, pot thermal probe, fritted glass dispersion tube and Dean-Stark trap with water-cooled condenser. Di-t-butylhydroxytoluene (BHT) (4.19 g, 3.50 wt %) and toluene (960 g) were charged to the reactor. Stirring was initiated and potassium carbonate (109.1 g, 7.67 mol) was added. The slurry was heated to 80° C. with an O2/Ar sparge and then methacrylic acid (119.9 g, 1.40 mol) was added dropwise at 100° C. over 2 hours. Carbon dioxide gas evolution was observed, and water was removed by the Dean-Stark trap under refluxing conditions. An azeotrope was observed starting at 90° C. After removing all water byproduct, tetrabutylphosphonium chloride (50% in toluene) (29.5 g, 0.031 mol) and 3-chloropropyldimethylethoxysilane (240.0 g, 1.33 mol) were added to the flask. The reaction mixture was heated at reflux for 3 hours and then cooled to room temperature. The reaction mixture was filtered. The filtrate was concentrated in vacuo and 5 wt % phenothiazine was added. The product was then purified by wiped film evaporation at 0.6-0.7 mmHg vacuum, with a 64-5° C. jacket temperature and a cold finger temperature of 30° C. with a product:residue split of 4:1 to afford the final product, 3-methacryloxypropyldimethylethoxysilane, as a clear colorless liquid (202.6 g, 66.2%).