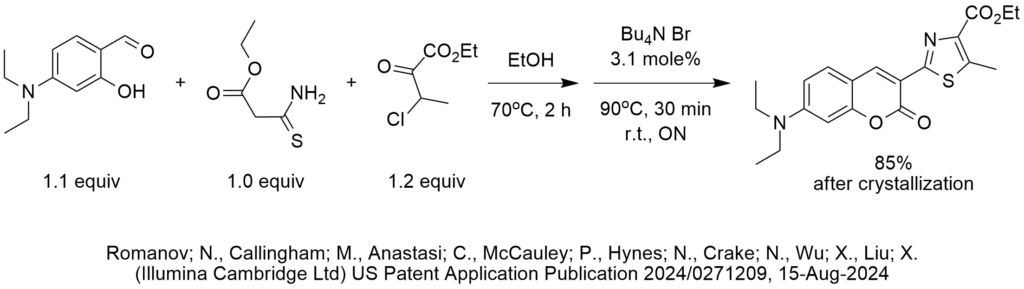
We came across a condensation reaction this month that uses TBAB (tetrabutylammonium bromide) as a catalyst at a loading of 3.1 mole%. The reaction is shown in the diagram.
However, it appears that either there is a missing reactant, such as a base to promote one of the condensation steps, or it is not clear what role TBAB could be playing in this reaction.
What do you think?
The reaction was performed in two steps. First the three reactants were mixed and heated at 70 deg C in ethanol for 2 hours. Then the TBAB was added to complete with additional 0.5 hour at 90 deg C and overnight at r.t.
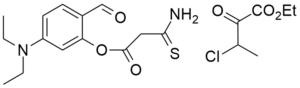
One possibility is that heating in ethanol facilitates a condensation reaction between the p-diethylaminosalicylaldehyde and the thioamide, resulting in the formation of an intermediate such as that shown in the next diagram. Another possibility is the formation of an imine from the aldehyde and the nucleophilic thioamide, though it is not clear how that would lead to the reported structure of the product.
If we assume that the intermediate is that shown in the second diagram, then TBAB could possibly catalyze a condensation induced by attack on the aldehyde by an anion formed by deprotonation of the methylene group in the beta diketone-thioketone IF a base was present, such as NaOH or potassium carbonate. PTC excels in the formation and nucleophilic attack of such anions. If this PTC catalyzed attack of the carbanion/enolate on the aldehyde, that would form the chromenone ring.
However, if base was not present, it is not clear what role the TBAB plays in these reactions. That is why we speculate that the addition of base was inadvertently omitted from the procedure.
At some point, the formation of a thiazole ring is likely driven by a nucleophilic attack on the carbonyl carbon of ethyl 3-chloro-2-oxobutanoate by an intermediate formed earlier. The sulfur atom in the thioamide participates in the cyclization, leading to the formation of a thiazole ring structure.
The isolated yield after crystallization is 85%.
Why do you think is happening in this reaction system?