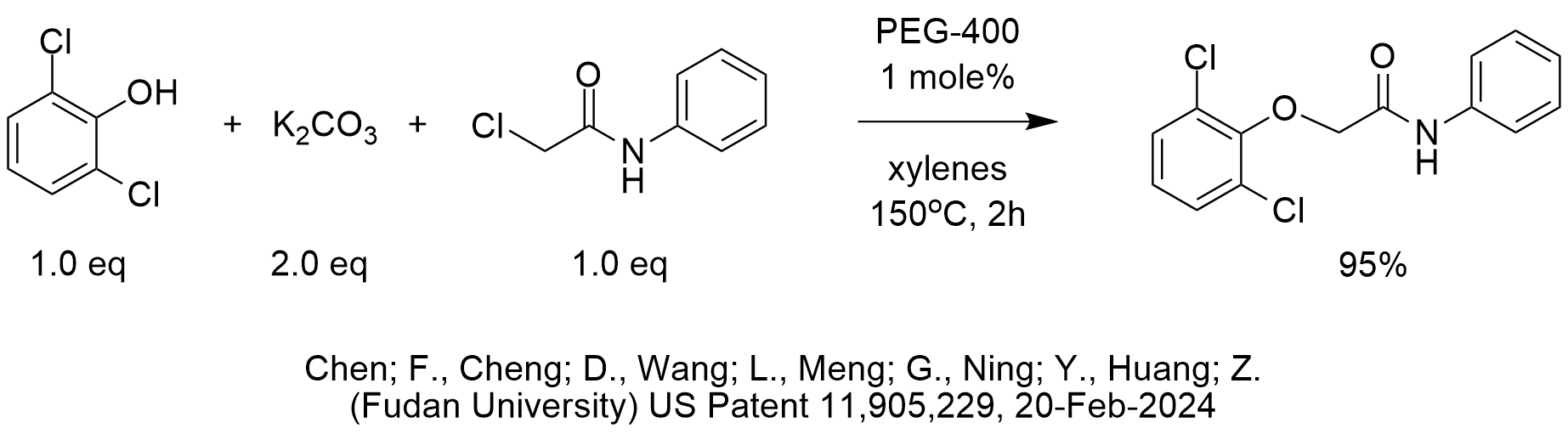
As we teach in the 2-day course “Industrial Phase-Transfer Catalysis,” polyethylene glycols serve as phase-transfer catalysts in a variety of applications. The reaction shown in the diagram is particularly interesting because it shows PEG-400 being very effective at a catalyst loading of only 1 mole%. We usually see PEG’s being used at higher levels and sometimes even as solvent.
PTC etherifications of phenols with alkyl halides rarely require temperatures much above 100 deg C but it is possible that in this case the weak nucleophilicity of 1,6-dichlorophenoxide might require more energy of activation. This temperature at 150 deg C is surprisingly, especially since the chloromethyl group is adjacent to an electron deficient carbonyl.
The yield is high without excess alkylating agent, so the reaction conditions obviously work well.
We are somewhat surprised to see the use of 2 equiv of potassium carbonate unless bubbling was observed at the high temperature. Once the first equivalent of carbonate deprotonates the acidic 1,6-dichlorophenol phenol (pKa might be about 7), any excess base could possibly deprotonate the N-H since even though it is less acidic than the O-H, it is still somewhat acidic due to it being conjugated to the aromatic ring and alpha to a carbonyl. Again, at this high temperature, carbon dioxide formation is possible from the inorganic bicarbonate byproduct. As we can see in this month’s PTC Reaction of the Month, only 1 equivalent of carbonate is required to deprotonate a phenol and achieve near quantitative yield, so that is why the use of 2 equivalents is surprising, especially when another acidic proton from an acidic N-H group is present that we know can alkylate under PTC conditions.
The inventors also screened PEG-600 at 1.5 mole% and obtained the same yield.
By the way, the structure of the product of this reaction [2-(2,6-dichlorophenoxy)-N-phenylacetamide] shown in the patent is incorrect. There is an extra methylene group in the structure in the reaction marked “condendsation.”
As a reminder, the mechanism of phase-transfer catalysis with PEG’s in etherifications like this one is that the non-bonding electrons of the 9 oxygen atoms of PEG-400 (or 13 oxygens of PEG-600), form a complex with the potassium cation. Since the potassium cation is associated with the dichlorophenoxide anion, the complex [PEG-K-dichlorophenoxide] is transferred from the solid-liquid interface into the xylenes phase and reacts with enhanced reactivity with the already activated electron deficient chloromethyl group.