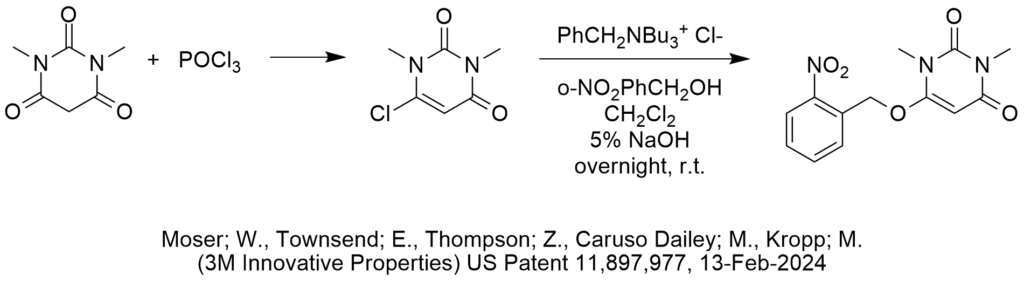
 There are two important points regarding the choices for the reaction conditions for the second reaction shown in the diagram which is obviously a PTC nucleophilic substitution. In this etherification, 1.5 equiv o-nitrobenzyl alcohol were reacted with N,N-dimethyl chlorouracil in the presence of 5 equiv NaOH diluted to 5% in water, methylene chloride as the solvent and 10 mole% benzyl tributylammonium chloride as the phase-transfer catalyst. The yield over two steps was 70% and the yield of the second step was 78% (based on the N,N-dimethyl barbituric acid).
There are two important points regarding the choices for the reaction conditions for the second reaction shown in the diagram which is obviously a PTC nucleophilic substitution. In this etherification, 1.5 equiv o-nitrobenzyl alcohol were reacted with N,N-dimethyl chlorouracil in the presence of 5 equiv NaOH diluted to 5% in water, methylene chloride as the solvent and 10 mole% benzyl tributylammonium chloride as the phase-transfer catalyst. The yield over two steps was 70% and the yield of the second step was 78% (based on the N,N-dimethyl barbituric acid).
One point is that when benzyl quats are used as phase-transfer catalysts in nucleophilic substitutions, the benzyl quat competes as an alkylating agent with the intended electrophilic substrate being attacked. In other words, whenever using a quat such as benzyl triethyl ammonium or benzyl tributyl ammonium, you must consider whether you will unintentionally form benzylated side products from the nucleophile.
We speculate that in this case, the avoidance of high temperature was keeping the benzylation of the nitrobenzyl alcohol low. The nitrobenzyl alcohol is used in excess and perhaps that masks some of the benzyl ether formation. Working at room temperature may be the cause for the long reaction time.
We usually prefer to avoid using benzyl quats in PTC nucleophilic substitutions. We discuss this in more detail in our 2-day course “Industrial Phase-Transfer Catalysis.”
A second point is that phase-transfer catalysis enhances the reaction between methylene chloride and NaOH to produce formaldehyde. We speculate that the high dilution of the NaOH may have been chosen to minimize formaldehyde formation and possibly also hydrolysis of the chlorouracil. We are somewhat surprised that the dilution of 4 g NaOH in 80 mL water afforded a base strong enough to perform the desired etherification, but the high yield is proof that these reaction conditions work.

