When one attempts to use PTC to hydrolyze an alkyl chloride directly to the corresponding alcohol by reaction with hydroxide, one usually obtains the dialkyl ether in high yield which is obviously undesirable. The reason for this is that when hydroxide is used under PTC conditions and can act as a base or as a nucleophile, it acts as a base. Once the first molecule of alkyl chloride reacts with hydroxide to produce the alcohol, the alkoxide is formed to some degree in equilibrium with the remaining hydroxide. At that point, the phase-transfer catalyst typically pairs selectively with the alkoxide instead of the hydroxide, even if the hydroxide is in great excess and the next reaction is usually the attack of the alkoxide paired with the quat on the alkyl chloride already in the organic phase. This forms the ether and it is often the case that there is only a low steady state concentration of the alcohol (or alkoxide) in the reaction mixture.
However, there is an effective method to leverage advantages of phase-transfer catalysis to perform this “hydrolysis.” This is done in two consecutive PTC steps that are [1] esterification and [2[ hydrolysis.
Since PTC excels in esterification, the first step usually reacts acetate or formate with the alkyl chloride to form the acetate or formate ester. In the second step, the acetate or formate is easily hydrolyzed to the alcohol by hydroxide. This 2-step process avoids the presence of an alkoxide and an alkyl halide that would lead to ether formation, so high yield of the 2-step “hydrolysis” can be achieved.
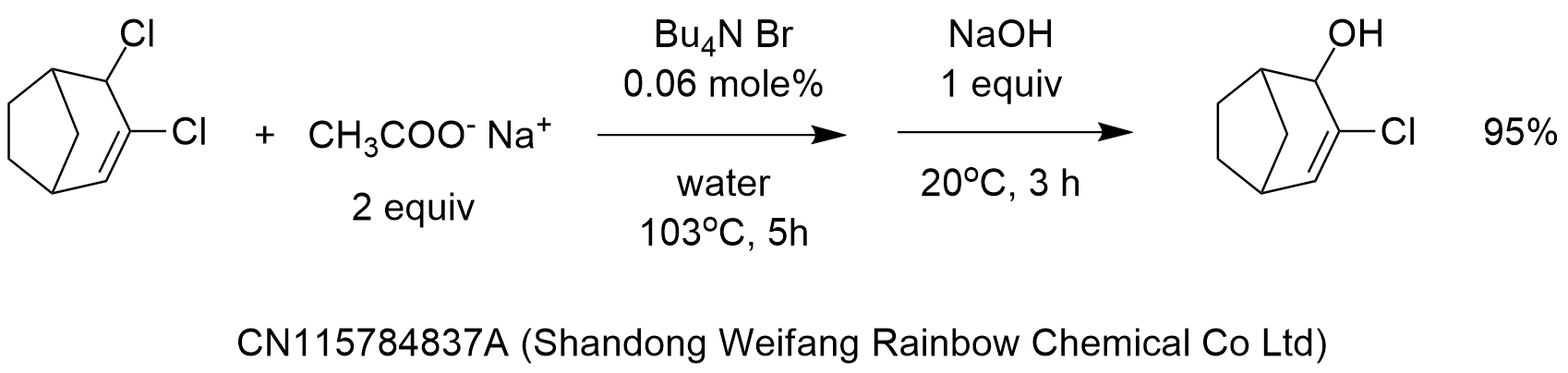
The procedure reported in the patent CN115784837A (Shandong Weifang Rainbow Chemical Co Ltd) for the 2-step “hydrolysis” shown in the diagram is as follows: “A mixture of 3, 4-dichlorobicyclo [3.2.1] -2-octene (88.5 g,0.5 mol), water (200 ml), sodium acetate (82.0 g,1.0 mol) and tetrabutylammonium bromide (0.1 g) was raised to 103 ℃ for a reflux reaction for 5h. Cooling to 20 ℃, adding sodium hydroxide (20.0 g,0.5 mol), keeping the temperature for reaction for 3h, cooling, extracting the mixture by dichloroethane, layering without generating floccules, drying the organic phase by sodium sulfate, and distilling to obtain 78.9g of yellow oily matter, the purity of a target product is 95.5% (external standard method); the yield was 95.0%.”
PTC Organics Inc has highly specialized expertise in performing the conversion of alkyl halides into the corresponding alcohols using acetate and formate. If your company needs to perform this conversion with low-cost high-performance green chemistry, now contact Marc Halpern of PTC Organics to explore integrating our highly specialized expertise with your commercial development or optimization program.