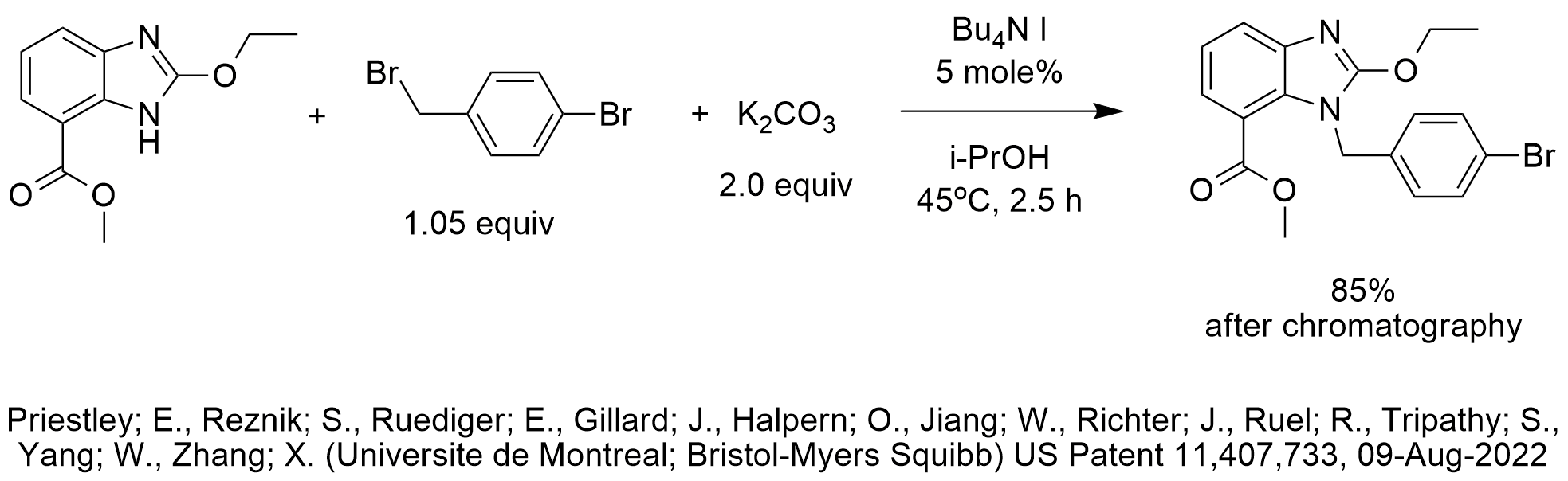
There are several interesting aspects to this patent. The first has nothing to do with chemistry. After drawing the reaction diagram, I was adding the names of the inventors and it was then that I first discovered that my son is one of the inventors. For obvious reasons of confidentiality and to avoid boring my grandchildren, we never talk about chemistry when we meet.
Getting back to chemistry…PTC excels in N-alkylations and is probably the most commonly performed PTC reaction after etherification since PTC almost always delivers high performance for etherifications and N-alkylations of N-H groups with pKa’s of 23 or less.
In this case, the pKa of the N-H of non-substituted benzimidazole is 12.8. The pKa of carbonate in water is 10.3. Under PTC conditions, bases act with enhanced basicity, often with basicity higher by several orders of magnitude than measured in water. So, the choice and use of potassium carbonate for this deprotonation under PTC conditions is very reasonable.
The use of 2 equiv potassium carbonate likely assures that the inorganic byproduct is potassium bicarbonate which minimizes formation of the water and carbon dioxide. The extra equivalent of potassium carbonate can also adsorb any water that my be formed or be present. The “hiding” of water enhances nucleophilicity of the imidazole N-anion.
While we usually prefer avoiding alcohols as solvents for PTC-base reactions, the steric hindrance around the secondary alcohol likely does not interfere with the basicity for deprotonation and the nucleophilicity of the imidazole N-anion.
Tetrabutylammonium iodide was chosen as the phase-transfer catalyst likely to co-catalyst the reaction (Finkelstein) by forming the benzyl iodide in-situ. PTC-iodide co-catalysis further reduces the energy of activation in addition to the absence of water by choosing 2 equiv of potassium carbonate. For these reasons, it is not surprising to achieve high conversion at the mild temperature of 45 C and the short reaction time of 2.5 hours.
In the procedure, the inventors reported adding isopropanol after 1 hour of the 2.5 hour total reaction time. We speculate, but do not know, that may have been due to overcome fluidity issues as the reaction progresses (KHCO3/KBr replaces 1 equiv of K2CO3 and accumulates).
As we each in our 2-day course “Industrial Phase-Transfer Catalysis,” whenever scaling up a reaction using tetrabutylammonium iodide, we almost always recommend using tetrabutylammonium bromide and KI due to the combination of the high price of TBAI and the much high affinity of iodide versus bromide for pairing with the tetrabutylammonium cation.
Another noteworthy item in this patent is the use of a large amount of tetrabutylammonium bromide (2 equiv) as the bromide source with catalytic copper (II) bromide (1 mole%), 1.2 equiv t-butyl nitrite and 1.2 equiv CSA (camphorsulfonic acid?…not defined in the patent) to replace an amino group with bromide (3-amino-4-cyano biphenyl to 3-bromo-4-cyano biphenyl).
If your company performs many N-alkylations, you should conduct the 2-day course “Industrial Phase-Transfer Catalysis” in-house at your company so your process development chemists and engineers will learn how to choose optimal PTC conditions in the shortest time and minimal number of experiments to improve R&D efficiency.