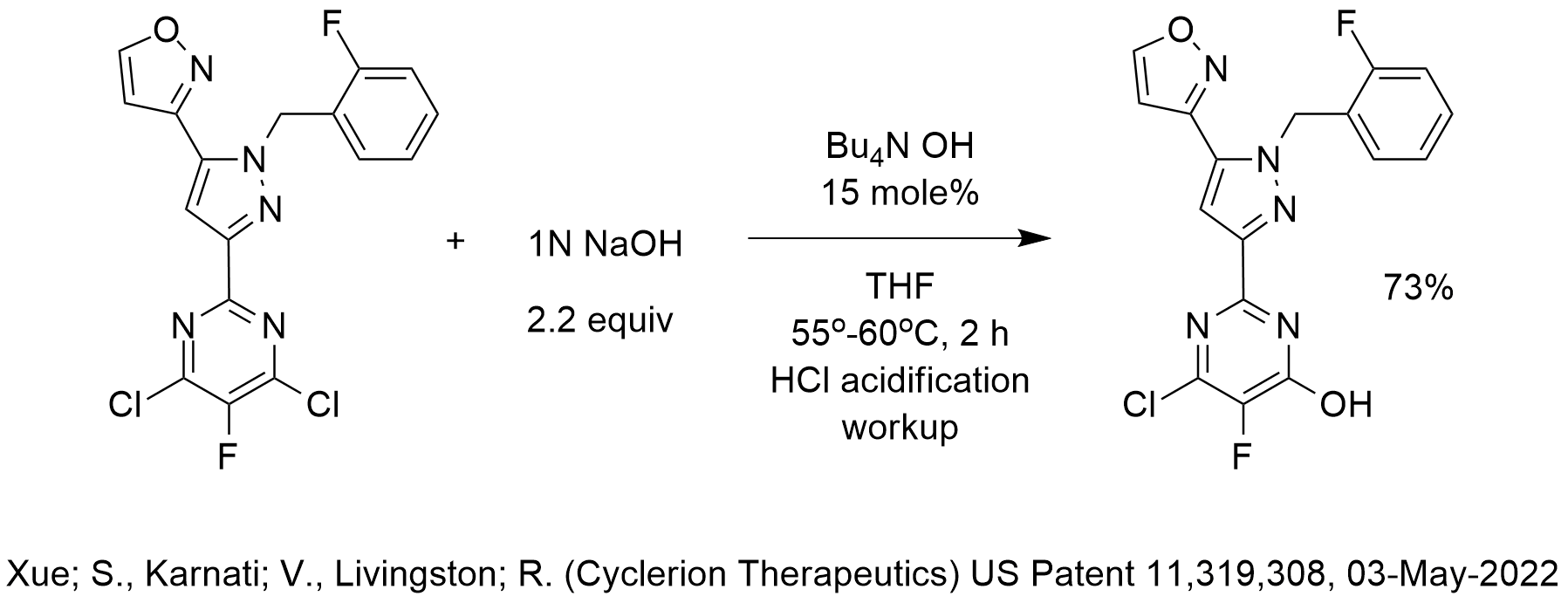
In the reaction shown, sodium hydroxide is used to “hydrolyze” one chlorine of a derivative of 4,6-dichloro-5-fluoropyrimidine. The reaction used tetrabutylammonium hydroxide as the phase-transfer catalyst and THF as the solvent.
If this reaction were to be scaled up for an economically feasible process, we would use a phase-transfer catalyst that is less expensive than TBA OH. We would also not choose a water-miscible solvent for the reaction (unless the product is not soluble in any water-immiscible solvent) in order to avoid yield loss during workup due to entrainment.
This reaction reminds me of a hydrolysis reaction performed commercially at Dow Chemical many years ago for the hydrolysis of symmetrical tetrachloropyridine (“sym-tet”) to form sodium trichloropyridinate (NaTCP) that served as a starting material for the large volume insecticide chlorpyrifos and herbicide triclorpyr. See for example Gatling, S.; Krumel, K.; (Dow Chemical) US Patent 4,814,448, 21-Mar-1989. Sterling Gatling and Karl Krumel were close friends of mine when I worked at Dow (my first industrial job! I learned to be a process chemist under their mentorship!) and they both received awards for outstanding phase-transfer catalysis process development.
The hydrolysis shown in the diagram is acidified to obtain the hydroxypyrimidine. The hydrolysis product used to produce NaTCP, the starting material for chlorpyrifos and triclorpyr, was used as-is in an aqueous slurry and was subsequently reacted with water sensitive compounds to produce these agrochemicals in high yield as we teach in our 2-day course “Industrial Phase-Transfer Catalysis.”
About Marc Halpern

Dr. Halpern is founder and president of PTC Organics, Inc., the only company dedicated exclusively to developing low-cost high-performance green chemistry processes for the manufacture of organic chemicals using Phase Transfer Catalysis. Dr. Halpern has innovated PTC breakthroughs for pharmaceuticals, agrochemicals, petrochemicals, monomers, polymers, flavors & fragrances, dyes & pigments and solvents. Dr. Halpern has provided PTC services on-site at more than 260 industrial process R&D departments in 37 countries and has helped chemical companies save > $200 million. Dr. Halpern co-authored five books including the best-selling “Phase-Transfer Catalysis: Fundamentals, Applications and Industrial Perspectives” and has presented the 2-day course “Practical Phase-Transfer Catalysis” at 50 locations in the US, Europe and Asia.
Dr. Halpern founded the journal “Industrial Phase-Transfer Catalysis” and “The PTC Tip of the Month” enjoyed by 2,100 qualified subscribers, now beyond 130 issues. In 2014, Dr. Halpern is celebrating his 30th year in the chemical industry, including serving as a process chemist at Dow Chemical, a supervisor of process chemistry at ICI, Director of R&D at Sybron Chemicals and founder and president of PTC Organics Inc. (15 years) and PTC Communications Inc. (20 years). Dr. Halpern also co-founded PTC Interface Inc. in 1989 and PTC Value Recovery Inc. in 1999. His academic breakthroughs include the PTC pKa Guidelines, the q-value for quat accessibility and he has achieved industrial PTC breakthroughs for a dozen strong base reactions as well as esterifications, transesterifications, epoxidations and chloromethylations plus contributed to more than 100 other industrial PTC process development projects.
Dr. Halpern has dedicated his adult life to his family and to phase-transfer catalysis (in that order!).


I am not sure if the term hydrolysis is proper . I would describe it as PTC promoted nucleophilic aromatic substitution instead
I agree