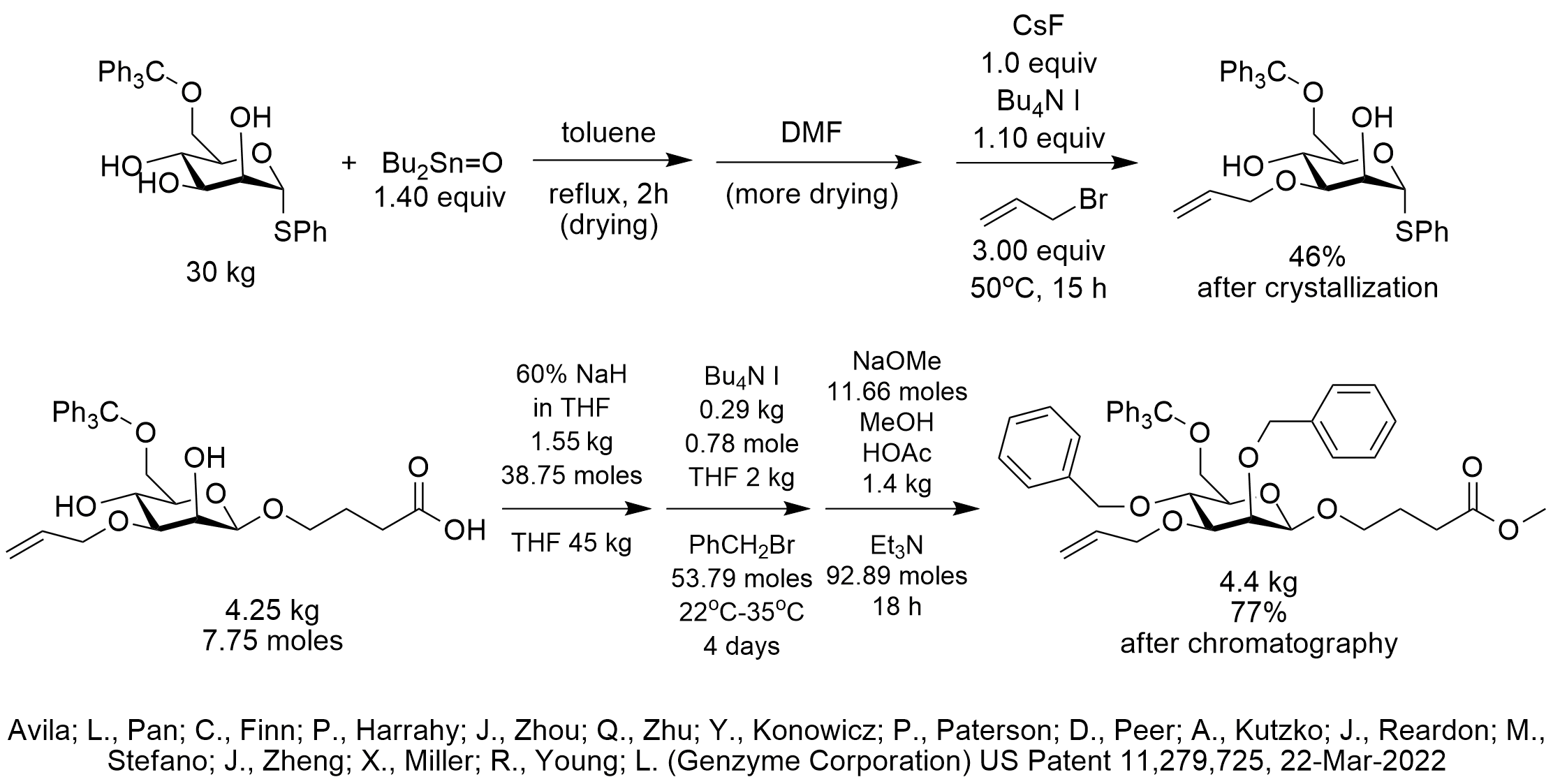
In this patent, tetrabutylammonium iodide was used as a source of iodide to activate alkyl halides for 5 reactions performed in DMF or THF. These included etherification using bromobutyrate methyl ester, selective monoetherification using allyl bromide and dietherification using benzyl bromide.
Dibutyltin oxide is particularly useful in directing regioselective O-alkylation for diols. In this case, dibutyltin oxide was used to achieve the monoetherification with the desired regioselectivity, in the presence of cesium fluoride as base. The alkylating agent was allyl bromide and was activated by iodide supplied by tetrabutyl ammonium iodide. We are not sure why so much TBAI was used since catalytic iodide would probably have been sufficient. This reaction started with 30 kg of the mannose derivative starting material.
In the dietherification using benzyl bromide, sodium hydride was used as the base. We speculate that such a strong base was used in dry THF to avoid esterification while producing the diether. Again, we are not sure why so much TBAI was used since catalytic iodide would probably have been sufficient.
These reactions were not phase-transfer catalysis since they used more than stoichiometric tetrabutyl ammonium iodide. We recommend screening the use of catalytic tetrabutyl ammonium bromide and catalytic potassium iodide to perform these etherifications on the multikilogram scales used in these reactions.
When your company needs to scale up etherifications, contact Marc Halpern of PTC Organics to benefit from the most highly specialized expertise in industrial phase-transfer catalysis for PTC-base reactions, especially etherification.
About Marc Halpern

Dr. Halpern is founder and president of PTC Organics, Inc., the only company dedicated exclusively to developing low-cost high-performance green chemistry processes for the manufacture of organic chemicals using Phase Transfer Catalysis. Dr. Halpern has innovated PTC breakthroughs for pharmaceuticals, agrochemicals, petrochemicals, monomers, polymers, flavors & fragrances, dyes & pigments and solvents. Dr. Halpern has provided PTC services on-site at more than 260 industrial process R&D departments in 37 countries and has helped chemical companies save > $200 million. Dr. Halpern co-authored five books including the best-selling “Phase-Transfer Catalysis: Fundamentals, Applications and Industrial Perspectives” and has presented the 2-day course “Practical Phase-Transfer Catalysis” at 50 locations in the US, Europe and Asia.
Dr. Halpern founded the journal “Industrial Phase-Transfer Catalysis” and “The PTC Tip of the Month” enjoyed by 2,100 qualified subscribers, now beyond 130 issues. In 2014, Dr. Halpern is celebrating his 30th year in the chemical industry, including serving as a process chemist at Dow Chemical, a supervisor of process chemistry at ICI, Director of R&D at Sybron Chemicals and founder and president of PTC Organics Inc. (15 years) and PTC Communications Inc. (20 years). Dr. Halpern also co-founded PTC Interface Inc. in 1989 and PTC Value Recovery Inc. in 1999. His academic breakthroughs include the PTC pKa Guidelines, the q-value for quat accessibility and he has achieved industrial PTC breakthroughs for a dozen strong base reactions as well as esterifications, transesterifications, epoxidations and chloromethylations plus contributed to more than 100 other industrial PTC process development projects.
Dr. Halpern has dedicated his adult life to his family and to phase-transfer catalysis (in that order!).


I am not sure if one would call these as PTC reactions as most of these reagents are soluble one one phase. Although I have not read the whole patent I believe the ester is produced ” excess Benzyl bromide used” during the formation of the ethers but was converted to the methyl ester by transesterication” with vast excess of Sodium methoxide used in the last step.
It looks like a sloppy calculation where excess reagents were used at a time we emphsize green chemistry.
Best wishes