The purpose of this patent is to describe a fluoroalkylating agent intended to avoid cost, safety and environmental drawbacks associated with other fluoroalkylating agents, especially those associated with CHF3 and Freons. The fluoroalkylating agent is di-N-methyl benzimidazole (quaternized at one of the nitrogen atoms) containing a trifluoromethyl group in the 2-position (between the two ring nitrogen atoms).
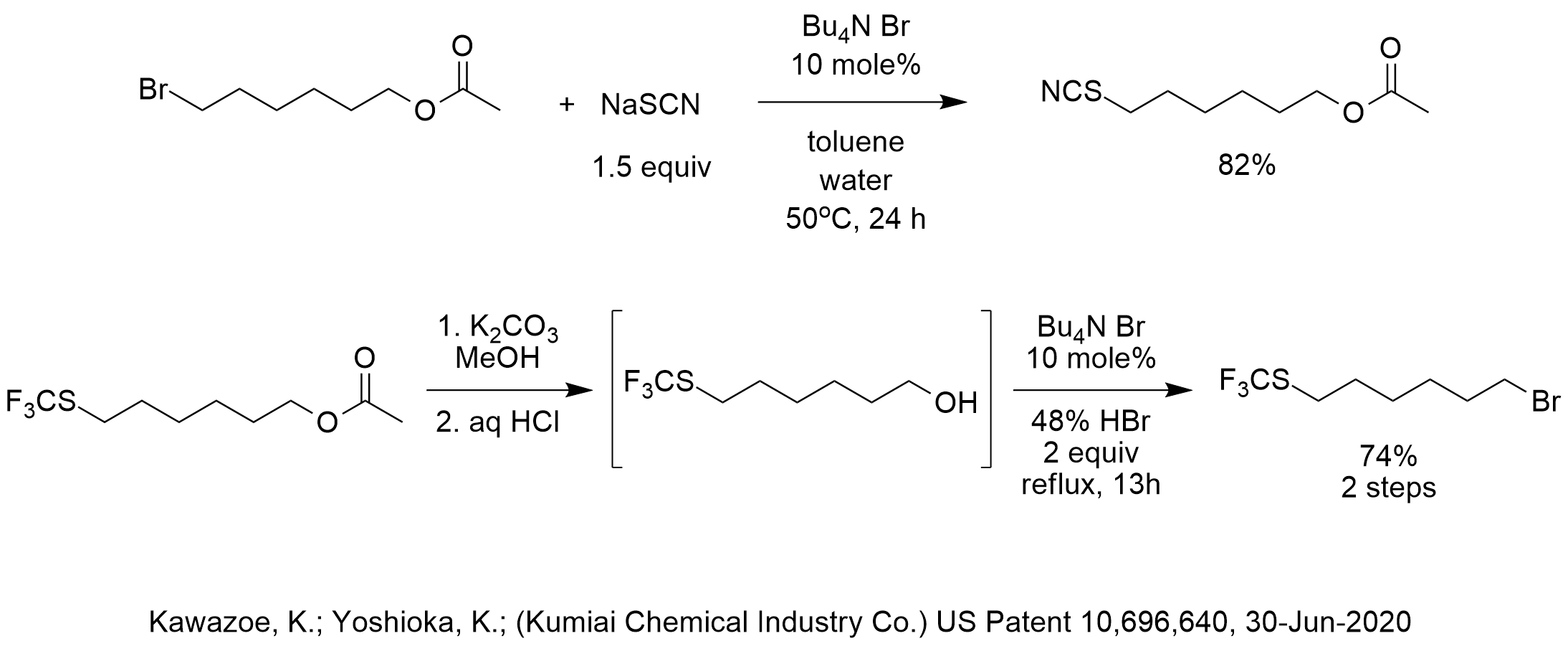
In order to prepare the starting material for the fluoromethylation reaction, this patent describes the PTC displacement of bromide with thiocyanate. The classic historical report of PTC thiocyanation was in the original PTC patent by Starks, US Patent 3,992,432. The Starks patent gave 100% conversion at 88-105 C using Aliquat 336 as the phase-transfer catalyst in toluene as the solvent using 4 equiv NaSCN . As shown in the diagram, the Kawazoe and Yoshioka patent gave 82% yield at only 50 C using TBAB as the phase-transfer catalyst in toluene as the solvent using 1.5 equiv NaSCN. It would be reasonable to assume that this PTC thiocyanation could be further optimized to achieve higher yield if scaled up.
Also in this patent, tetrabutylammonium bromide was used for another reaction that we don’t see very often but which is very effective and that is the transfer and reaction of HBr. In such PTC-acid reactions, the hydrogen of the HX acid associates with the halide of the quat salt through hydrogen bonding and the QBr-HBr complex distributes into the organic phase for reaction. In this case, the PTC-acid reaction replaced the hydroxyl of 6-trifluoromethylthiohexan-1-ol with bromide. The yield of the two steps, including the hydrolysis of the acetate in the previous step, was 74%.
It is interesting to consider that if the reagent trifluorothiomethanol would be available, PTC could have been used to directly convert the bromohexyl acetate to trifluorothiomethylhexyl acetate in one step without using the fluoroalkylating agent which is the subject of the patent.
It is also interesting to note that formation of the acetate using acetic anhydride used the highly toxic DMAP and could probably have also been replaced by PTC and a base such as sodium carbonate, as we teach in the 2-day course “Industrial Phase-Transfer Catalysis.”
If your company needs to develop and commercialize low-cost high-performance green chemistry processes for nucleophilic displacements such as thiocyanation or acid reactions such as HCl or HBr additions to alkenes or conversion of alcohols to alkyl halides, now contact Dr. Marc Halpern of PTC Organics to explore joint process development.