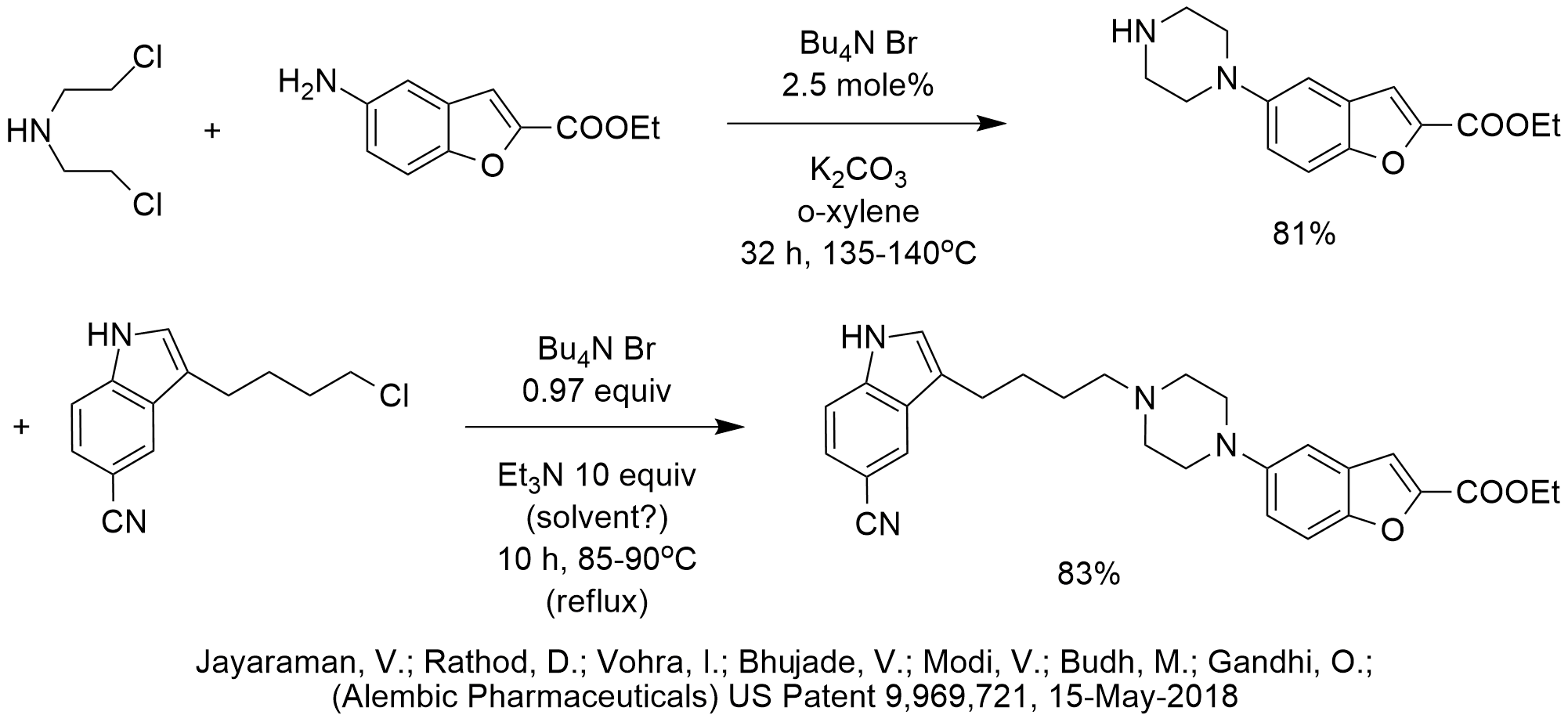
This patent describes the synthesis of Vilazodone on the 100 g scale. There are several interesting items of note for the phase-transfer catalyzed reactions reported.
First, there appear to be two consecutive PTC N-alkylations performed. The first N-alkylation is a classical phase-transfer catalyzed reaction and uses 2.5 mole% TBAB as the catalyst. For some unexplained reason, the second N-alkylation uses nearly a full equivalent of TBAB. Since the leaving group in both reactions is chloride, we see no possibility for catalyst poisoning, like we would if iodide or tosylate was the leaving group.
We are also surprised by the use of so much triethylamine in the second N-alkylation (10 equiv!). Its volume suggests that it is being used as solvent, not just base or as an activator of the C-Cl bond.
One advantage that is characteristic of many PTC reactions is the ability to maintain the ester intact when using base.
We would have expected a shorter reaction time and/or a lower temperature for the first PTC N-alkylation, especially since the bromide of TBAB should be co-catalyzing the reaction by forming the bromoalkyl amine in-situ from the chloroalkyl amine.
In fact, TBAB is often not stable when heated a day or more at a temperature of 135 C. Perhaps that is a factor in the long reaction time. Then again, when calculating the number of moles of potassium carbonate being used in the first N-alkylation and taking into account that the bis[chloroethyl]amine is introduced as the dihydrochloride, there doesn’t appear to be a whole lot of base remaining to neutralize all of the HCl present or generated during the reaction.
Another interesting point is that PTC is very effective for performing N-alkylation of the NH group of the indole. That may be affecting the choice of triethylamine as base and solvent to control the alkylation at the less acidic and more nucleophilic NH of the piperazine versus the more acidic and less nucleophilic NH of the indole.
In cases like these, it is not always clear if the inventors are missing something due to lack of specialized expertise in PTC or whether we are missing something without the benefit of experience with this reaction. In all cases, one must view all PTC reports with a critical eye for the potential learning value.
If you need help understanding and optimizing your commercial PTC process in development or in production, now contact Marc Halpern of PTC Organics to improve process performance.