Three N-alkylations were published this month using tetrabutylammonium iodide and each used conditions that were surprising.
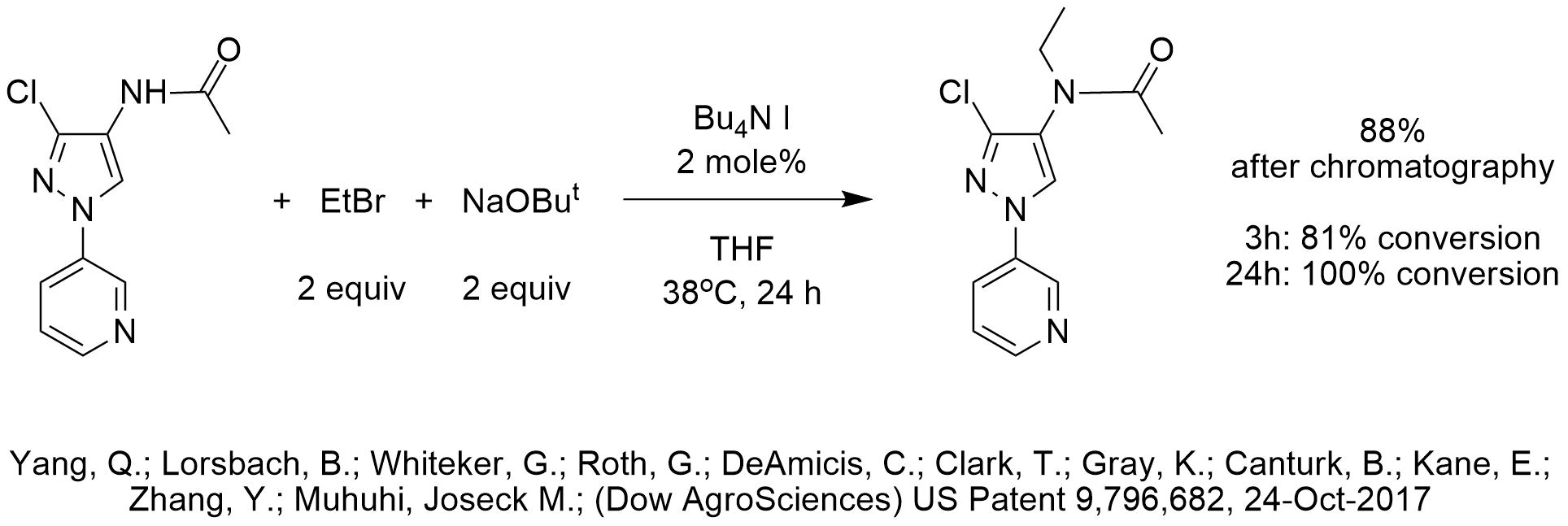
N-Ethylation of Acetamide Using TBAI
The inventors used the combination of tetrabutylammonium iodide catalysis to enable the N-ethylation of an acetamide using ethyl bromide that is much less expensive than ethyl iodide. The use of THF as the solvent may have been required in order to solubilize the polar reactant. We are not sure why the inventors chose sodium t-butoxide as base instead of the less expensive and easier to handle sodium hydroxide though the reason may have been solubility in THF. We speculate that a less polar solvent could have been used and form two phases with water during workup in which case PTC-NaOH conditions are known to be effective in alkylations of substrates with a pKa of 23 or less using ethyl bromide.
N-Alkylation of Indazole Using TBAI
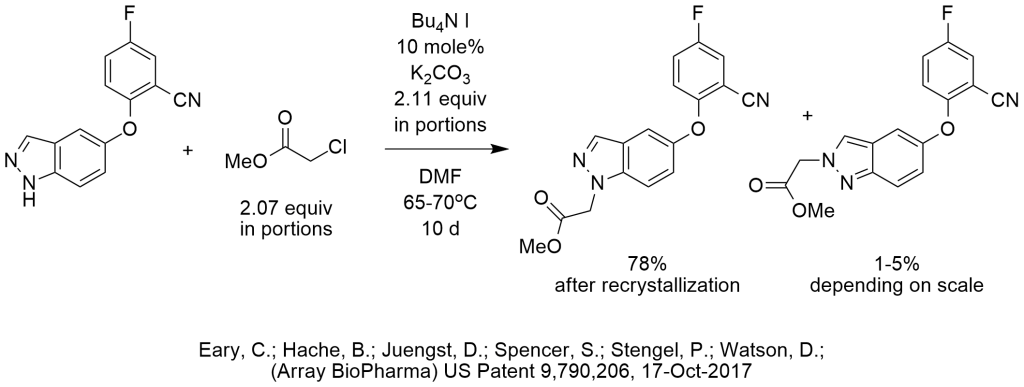
TBAI was used to activate methyl chloroacetate in the N-alkylation of an indazole. We are not sure why the inventors used such a polar solvent unless it was really necessary to dissolve the indazole. Again, we speculate that a less polar solvent could have been used and form two phases with water during workup in which case PTC-NaOH conditions are known to be effective in alkylations of substrates with a pKa of 23 or less using methyl chloroacetate.
When reading the procedure, it seems like the inventors initially expected the reaction to be complete by using 1.5 equiv of methyl chloroacetate and potassium carbonate. The extended reaction time was likely due to the multiple additions of alkylating agent and base after every analysis. We too would have expected the reaction to go to completion with the initial charge of 1.5 equiv methyl chloroacetate and potassium carbonate that also serves as a desiccant, when using PTC-iodide co-catalysis and a less polar solvent more convenient for solvent recovery.
The challenge in this reaction appears to be the selectivity of which nitrogen to alkylate.
N-Alkylation of Tetrahydropyrimidinone Using TBAI
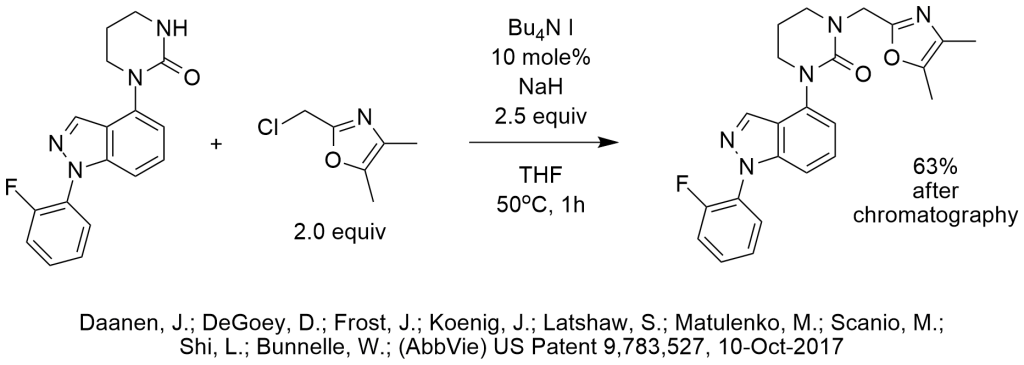
In this N-alkylation, sodium hydride may have been needed due to the high pKa of the N-H of the tetrahydropyrimidinone, so PTC-NaOH conditions were probably not feasible and the use of a less polar water immiscible solvent would not have provided advantage. As in the examples above, catalytic TBAI was used as an iodide catalyst to activate the chloromethyl group.
Choose PTC conditions like an expert to improve your N-alkylations! How? Now bring the excellent course “Industrial Phase-Transfer Catalysis” to your company and train your staff to improve numerous processes and increase R&D efficiency. Now contact Marc Halpern of PTC Organics.