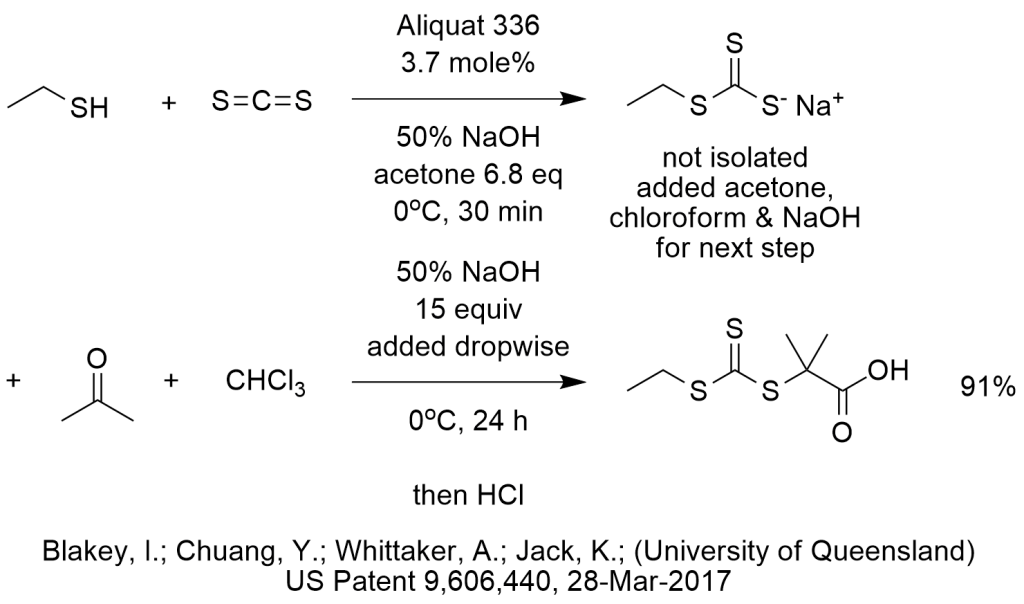
The diagram shows what appears to be consecutive PTC reactions with a good outcome. However, not all of the chemistry is obvious to this reviewer and we need your help to explain how these reactants lead to this product.
Please read the procedure shown below (provided in this patent) in order to figure out which, if any, of the PTC reactions shown below may be at work in this sequence, then contact Marc Halpern writing in the comments section your explanation of the reactions and source of each carbon atom. Thank you in advance for your help.
25 g NaOH (50 wt %) was added dropwise to a solution of ethanethiol (18.9 g), Aliquat 336 (4.9 g) and 150 mL acetone at 0 deg C. over 20 minutes. The reaction was then stirred for a further 10 minutes before adding carbon disulfide (18 mL) and the mixture was then diluted with 30 mL acetone dropwise over 20 minutes. After stirring for another 10 minutes, chloroform was added in one portion and then 120 mL NaOH (50 wt %) was added dropwise over 20 minutes. The resulting solution was then stirred at 0 deg C for 24 hours. Purification was performed by removing acetone, redissolving in 200 mL water and then adding 300 mL concentrated HCl(aq) while stirring rapidly in an ice bath. The sample was extracted with PET spirit then washed with water three times. The PET phase was collected and dried with magnesium sulphate (MgSO4). The solution was then filtered and concentrated by rotary evaporation. The product was recrystallized from PET spirit three times, resulting in bright yellow crystals. (91.2%) 1H NMR (400 MHz, CDCl3): δ=3.30 (q, 2H), 1.73 (s, 6H). 1.33 (t, 3H). 13C NMR (400 MHz, CDCl3): δ=207.18 (C=S), 177.35 (COOH), 55.49 (SCCH3), 31.26 (SCH2), 25.22 (CCH3), 12.85 (CH2CH3).
Phase-transfer catalysis excels in many nucleophilic substitutions and strong base reactions. The procedure provided is Example 2.2 in the patent and appears to start with one of PTC ‘s strengths which is the transfer and reaction of thiolates. In the first step, ethyl mercaptide is easily formed by neutralization with base and should easily react with carbon disulfide in acetone, aided by Aliquat 336 to produce the first intermediate that we assume is ethyl trithiocarbonate sodium salt. It is possible that this reaction works without PTC and that Aliquat 336 possibly reduces the energy of activation enough to keep the temperature low enough to avoid/minimize the ignition of carbon disulfide.
The next reactions are the ones in question. When looking at the placement of the carbon atoms, one may be able to suggest that the electron rich S-anion attacks the electron deficient carbonyl carbon of acetone. But, if so, then what? Is it possible that dehydration happens under basic conditions? Even if it is possible, then what?
PTC excels in carbene reactions in which dichlorocarbene is formed from chloroform and NaOH and adds to electrophiles. If the electrophilic dichlorocarbene is the active species, would it add to the double bond if the intermediate from the reaction of the S-anion with acetone dehydrated? If so, can the dichlorocyclopropane somehow be ring opened and hydrolyzed to the carboxylic acid.
Alternatively, is it possible that when the S-anion attacks acetone to form an O-anion, can the O-anion react with the dichlorocarbene then rearrange before hydrolyzing into the carboxylic acid?
These all seem to be strange speculations.
PTC-NaOH-chloroform is also known to convert alcohols to alkyl chlorides in high yield (with retention of configuration through an SNi mechanism). Can this be somehow involved in this reaction sequence?
Of course, PTC also excels in alkylation of ketones, so acetone could be deprotonated under these PTC-strong base conditions. But then what?
It is also possible that the structure of the product is drawn incorrectly. The structure drawn is consistent with another structure shown later in the patent and is also consistent with the compound name.
Your challenge is to figure out what reactions are happening here with which reactants and confirm or refute the structure of the product shown. While it is embarrassing to admit not understanding the chemistry in this sequence, I am not embarrassed to ask for help from smarter organic chemists. We understand phase-transfer catalysis extremely well, but we need your help to understand the chemistry.
Please submit your understanding of this chemistry using the comments section of the contact form and next month we will publish the best explanations and name the contributors. If you don’t want your name published, please let us know.

