A patent issued this month that describes a solution to an occasional challenge encountered in PTC reactions which is when a PTC reaction slows down or stalls more than expected based on simple kinetics or for other reasons.
When PTC reactions actually stall, it is often due to decomposition of the phase-transfer catalyst. This can be diagnosed by simply adding more phase-transfer catalyst which jump starts the reaction and drives it to completion.
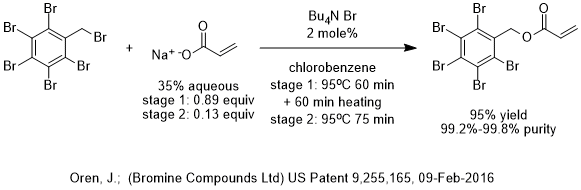
In the case of US Patent 9,255,165, the reaction is conclusively shown (using comparative examples and control experiments) to be slowed down by the accumulation of bromide leaving group that competes with the acrylate anion that is the nucleophilic reactant.
As we teach in our 2-day PTC course, quaternary ammonium phase-transfer catalysts have different affinities toward different anions. For example quats preferably associate about 10 times more with bromide than with chloride and about 1,000 times more than with fluoride. A lower alkyl carboxylate anion such as acetate has an affinity toward quats that is about 10 times less than chloride or 100 times less than bromide.
As a result, as a PTC reaction progresses that has a bromide leaving group and a carboxylate anion with low number of carbons, the accumulating bromide provides more and more competition for association with the quat and slows down the reaction for thermodynamic association/extraction reasons that go beyond the normal kinetic reasons of diminishing concentration of the reacting anion.
In this case, the bromide does not act as a true catalyst poison that would totally shut down the reaction, but it slows down the transfer of acrylate so much that it is worthwhile to physically remove the bromide from the system and replace it with fresh acrylate.
The invention disclosed by the inventor is to carry out the PTC nucleophilic esterification reaction between sodium acrylate and pentabromobenzyl bromide in chlorobenzene and TBAB as the phase-transfer catalyst, in batch mode, to rather high conversion of 75% to 95% within an hour, separate the aqueous phase and replace it with fresh aqueous sodium acrylate. The second stage reaction proceeds to completion in another 60-75 minutes.
The process is also described in continuous mode.
Such a solution to this problem is obvious to a PTC expert who is well aware of the relative affinities of various anions to various quaternary ammonium cations. Now contact Marc Halpern of PTC Organics if you want to benefit from the most highly specialized expertise in phase-transfer catalysis to achieve your process development goals in the shortest development time.